Putting East London at the forefront of healthcare innovations
Clinical trials are an essential step in getting the most promising new therapies available to those who need them most. A Clinical Research Facility is where this important work happens, with some of these spaces designed to host early-phase trials—when treatments are first made available to a small, select group.
These clinical trials have enormous value for participants, clinicians and the scientific community. But until now, there hasn’t been anywhere in East London suitable for early-phase clinical trials. This has made it harder for patients to gain potential benefit from new drugs, has limited options for clinicians and for locally-led research.
But this is about to change.
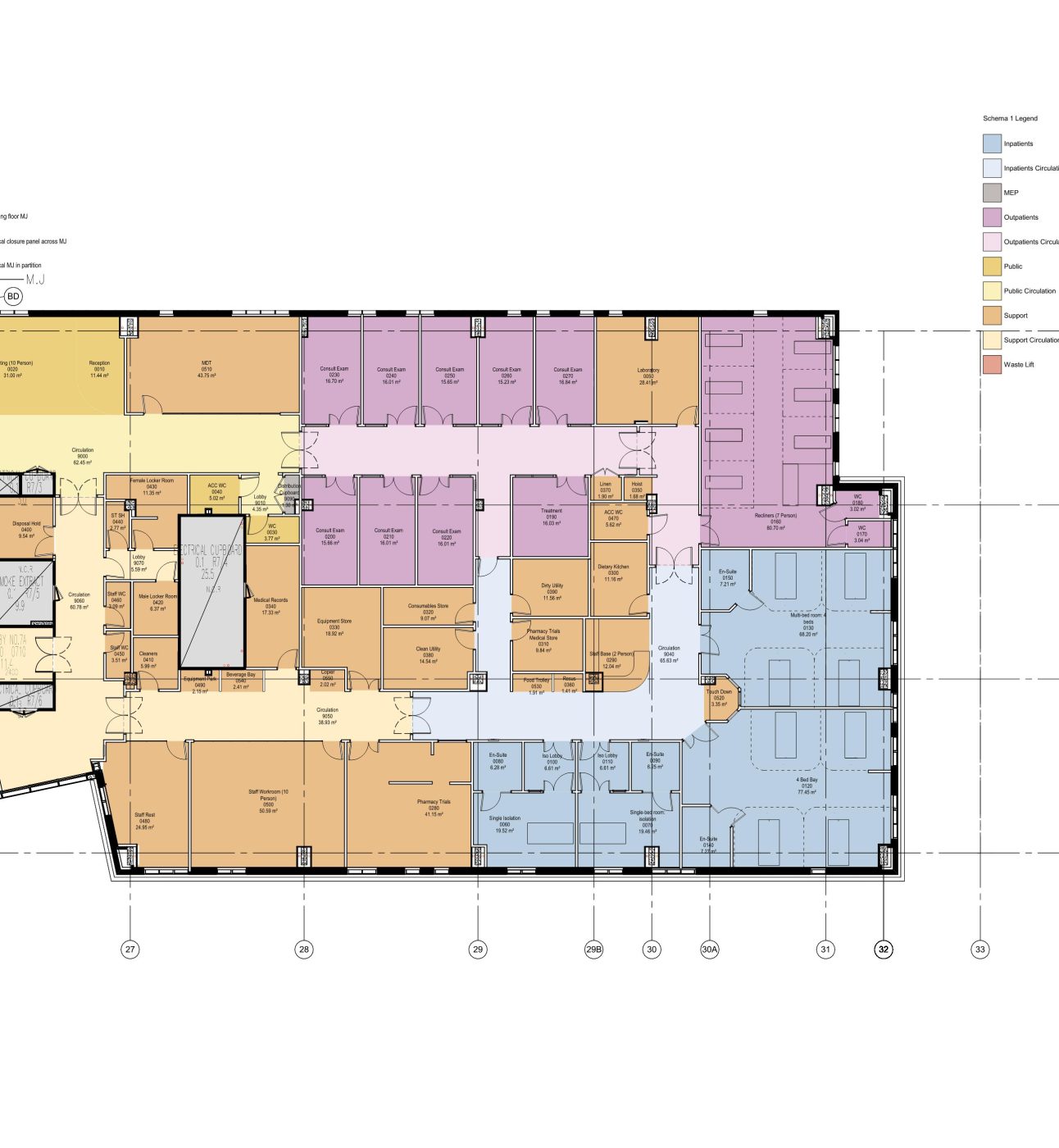
We’re partnering with Barts Health NHS Trust and Queen Mary University of London to build a new, early phase Clinical Research Facility at The Royal London Hospital in Whitechapel. And our generous donors are helping to make it happen. This new facility will enable more life-changing research for people like Suresh.
What will the new Clinical Research Facility enable: our vision
- More trials, spanning more diseases, particularly those where our local population has higher incidence and/or poorer outcomes
- Improved local access to novel therapies
- Increased opportunities for clinicians to engage in research, something which we know improves patient outcomes across the board
Benefitting from diversity
We still know too little about how effective particular drugs may be in patients from different ethnic backgrounds. This problem can be compounded when clinical trials for new treatments don’t adequately reflect the make-up of the likely patient population. But by conducting more early phase trials in East London—and by ensuring that eligible patients from all of our many communities can participate—we will be helping to ensure better outcomes: for our patients, for London, and for the world.
The project recently received the endorsement of a £1 million National Institute of Health and Care Research (NIHR) Clinical Research Facility infrastructure award, allowing us to join the national NIHR CRF network.